Common Light Chain Antibody
Kyinno's Bispecific Antibody Platform with Common Light Chain Mice
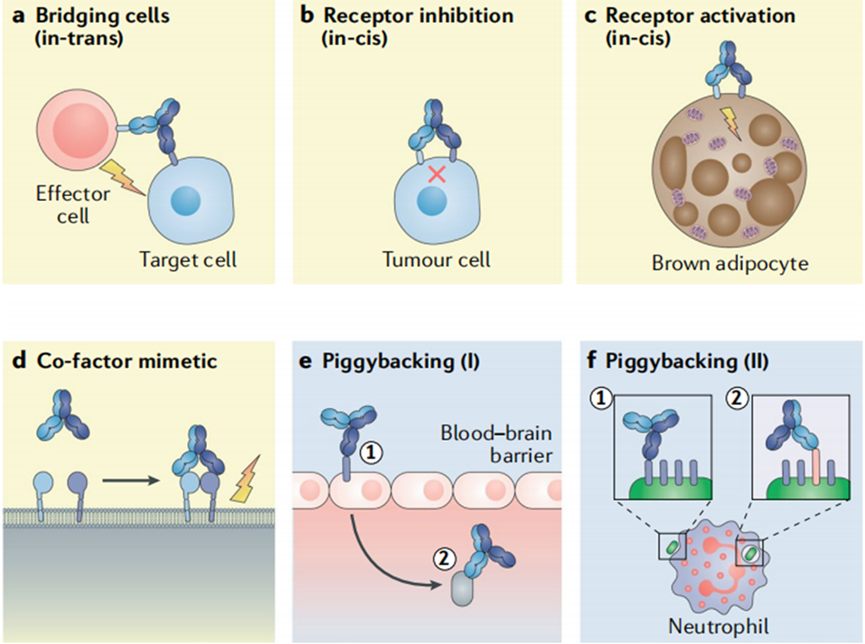
Bispecific antibody (BsAb) is engineered antibody that can simultaneously bind to two antigens or epitopes. Due to this multifunctionality, it have gradually become the focus of antibody drug development. BsAb can activate immune cells by connecting them to tumor cells via molecular bridging; it can also block the interation between the two antigens and their corresponding ligands, leads to the inhibition of the antigen-mediated signaling pathways which were related to tumor cell proliferation, differentiation and metastasis; bispecific ADC (Antibody-Drug-Conjugated), which was building on the BsAb, has been an important direction for the future development of ADC, with the high specificity, high endocytosis and low cytotoxicity. As of August 2023, twelve BsAbs were approved and the global market size was $ 5.8 billion. The expected market will reach to at least tens of billions by the year 2030.
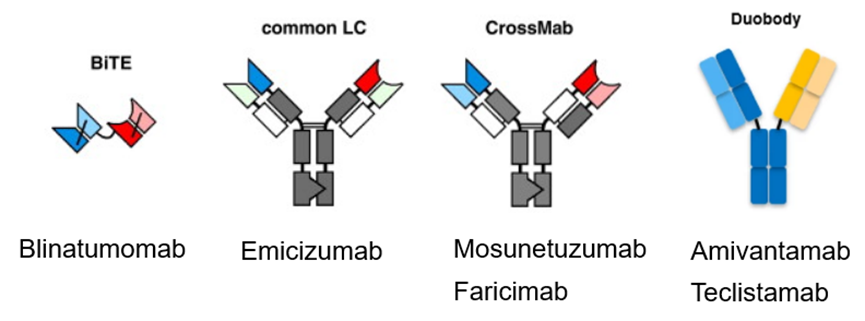
To support the development of bispecific antibodies, Kyinno has successfully developed the Common Light Chain (KY-CLCTM) Mouse technology platform. The KY-CLC-mouse features no lambda (λ) light chain expression and fully identical kappa (κ) light chains, which offer excellent solubility and stability (protected by patent). KY-CLC-mouse enables rapid and efficient screening for high-affinity, highly specific antibodies against all validated targets. Bispecific antibodies assembled using this platform are comparable to monoclonal antibodies in terms of expression, purification, stability, and other critical attributes.
We welcome collaboration to explore the opportunities this platform provides.

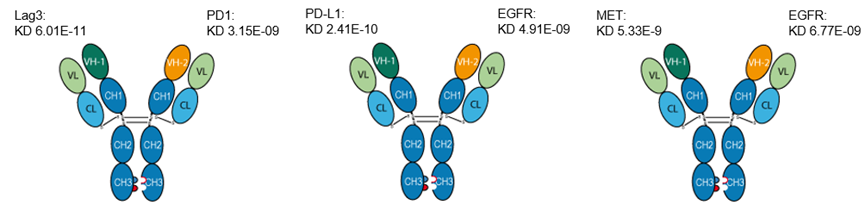
Affinity tests of LAG3/PD1, PD-L1/EGFR, MET/EGFR bispecific antibodies:
Case Study
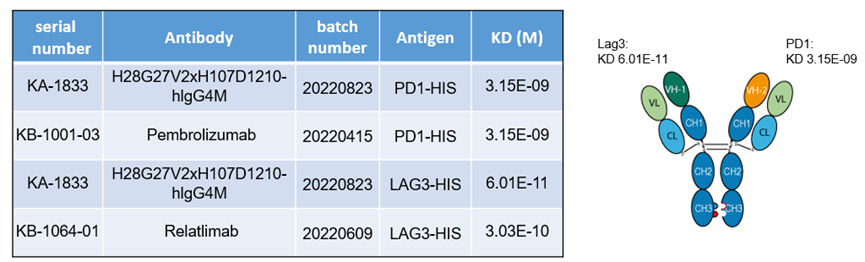
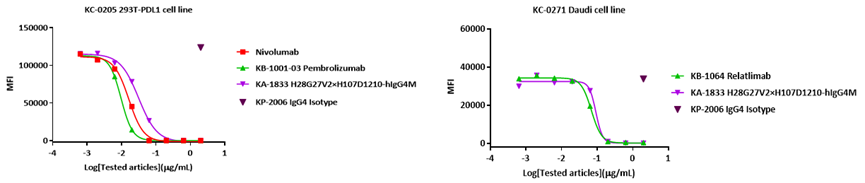
1. LAG3/PD1 bispecific antibody
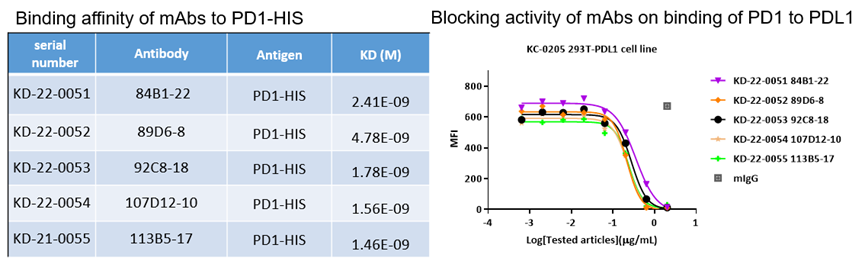
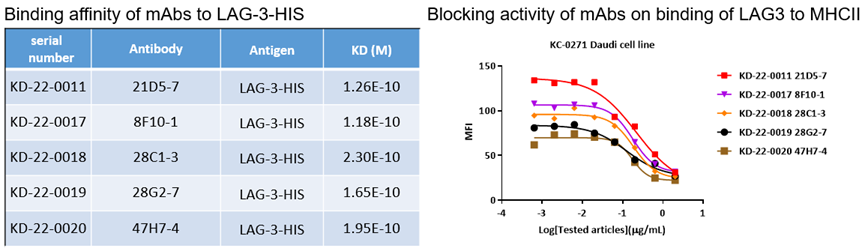
Binding affinities of bispecific antibody KA-1833 against PD1 and LAG3 to PD1-HIS and LAG3-HIS
2. Blocking activity of bispecific antibody KA-1833 against PD1 and LAG3
3. SEC-HPLC and SDS-PAGE

4. PD1
5. LAG3
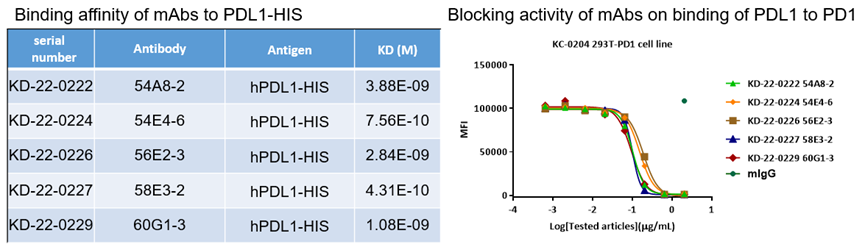
6. PDL1
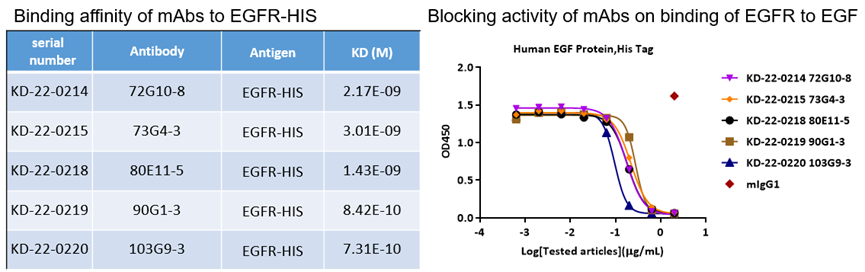
7. EGFR
7. CD47

References:
- Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019 Aug;18(8):585-608.
- Bispecific antibodies targeting dual tumor-associated antigens in cancer therapy. Review – Cancer Research. 28 September 2020